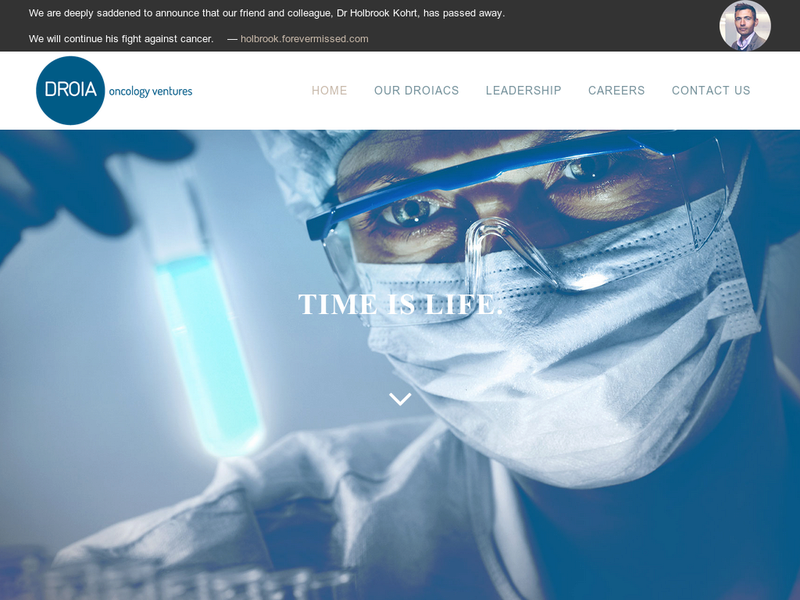
About DROIA
DROIA is a specialist investor, exclusively focused on the development of oncology therapies. We are dedicated to making a difference in the fight against cancer. We apply a unique investment model that helps accelerating development within our portfolio companies, the DROIACs. Through this we aim to bring promising therapies to patients faster, increasing both patient benefit as well as shareholder value. Hence our name: Double Return On Investment, Accelerated: DROIA
DROIA was created in 2011, is funded by private investors, and operates from offices in Luxemburg and Brussels. To day we made 5 investments with immune therapy and tumor cell metabolism as main focus areas within the oncology field. We invest in early stage drug development companies with solid preclinical proof of concept. Our target is to fund our companies at least up to human proof of concept (phase 1b / 2a). Our investment decisions are driven by research data and based upon the breakthrough potential as expressed by the novelty and potential patient benefit of a candidate therapy.
DROIA is a hands-on investor and actively participates in the development projects of the DROIACs. To this end, we have an expert team within DROIA with extensive experience in the main aspects of oncology, drug development and entrepreneurship. We help the DROIACS by providing not just financial means, but also with assistance and advice on scientific, preclinical, clinical & CMC development, IP & regulatory, licensing & transactions, and organization & administration. Our network of oncology world leaders gives us access to top-level institutions around the world, allowing our companies to benefit from a global knowledge base. DROIA is not a closed end fund, allowing us to maintain a long-term vision and continued support for the DROIACs.
Estrategia inversión DROIA
-
Market preferences:
-
Location preferences:
Estretegía de inversión no pública.
Equipo DROIA 0
DROIA no tiene a su equipo agregado
Noticias DROIA 0
DROIA no tiene ninguna noticia disponible.